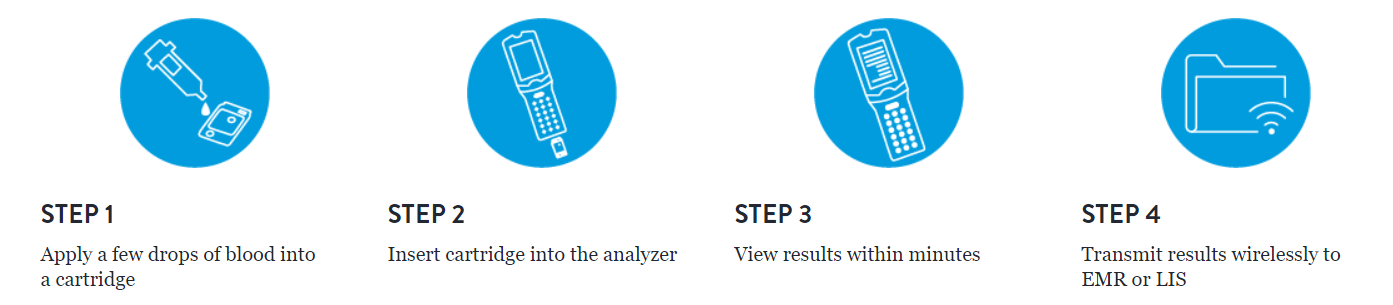
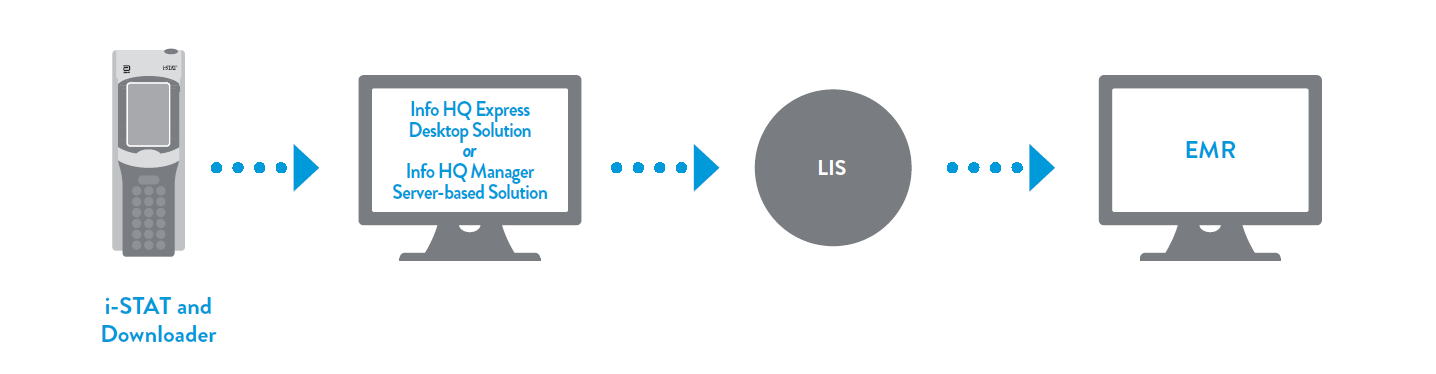
A handheld blood analyzer that delivers lab-quality, diagnostic results in minutes.
Lightweight, portable and easy to use, the i-STAT 1 blood analyzer operates with the advanced technology of i-STAT test cartridges. Together, they create the i-STAT System — a point-of-care-testing platform that provides healthcare professionals with diagnostic information when and where it’s needed.